Vitamin D and Multiple Sclerosis
> 800 IU/day (20 microgram/day) of Vitamin D3
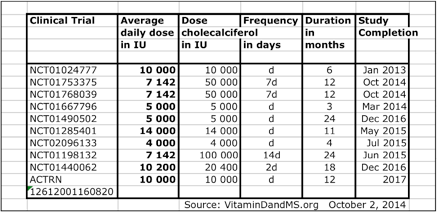
Overview of Clinical Trials with Vitamin D3 for patients with Multiple Sclerosis
Clinical Trial NCT Number, Average daily dose in IU, Dose of Cholecalciferol in IU, Dose Frequency, Duration of the trial in months, Date of Study Completion.
Peter A. Calabresi, Johns Hopkins University
Safety and Immunologic Effect of Low Dose Versus High Dose Vitamine D3 in Multiple Sclerosis
Dose: vitamin D3 400 IU/d versus 10 000 IU/d, for 6 months
Estimated Enrollment: 40
Start Date: March 2010
Completion Date: January 2013
NCT Number: NCT01024777
Brief summary:
The purpose of this study is to determine the safety and the immunologic effects of supplementation with low-dose and high-dose cholecalciferol (vitamin D3) in patients with multiple sclerosis.
Publication(s):
Samia Khoury, American University of Beirut Medical Center
Effect of Vitamine D Replacement on Immune Function and Cognition in MS Patients
Dose: low vitamin D (less than 25 ng/ml [62.5 nmol/L]) and normal vitamin D level (greater than 35 ng/ml [87.5 nmol/L])
Estimated Enrollment: 86
Start Date: August 2012
Completion Date: August 2014
NCT Number: NCT01952483
Brief summary:
Assessing the immune activation in MS patients deficient in Vitamin D and whether Vitamin D supplementation reverse the immune activation
Evaluating whether Vitamin D deficiency result in lower cognitive performance in MS patients and the effect of Vitamin D supplementation on reversing the cognitive impairment?
Publication(s):
AlJohara M AlQuaiz, M.D., King Saud University
Role of Vitamin D in Reducing the Relapse Rate in Patients With Multiple Sclerosis
Dose: 50 000 IU/w vitamin D3 versus placebo, for 12 months
Estimated Enrollment: 200
Start Date: January 2013
Completion Date: October 2014
NCT Number: NCT01753375
Brief summary:
Vitamin D3 supplementation reduces the incidence of multiple sclerosis.Although clinical cross-sectional studies have demonstrated vitamin D3 as a positive mediator in preventing relapses and disease progression, prospective randomized control trials are nevertheless necessary to confirm these statements and to determine the most efficacious, safe, and the minimum required doses. This hypothesis is going to be tested through a randomized triple blinded controlled trial in which after randomization, one group of patients will receive vitamin D and second group will receive placebo. Both groups are going to be followed in a similar way over a period of one year with follow ups at 4, 8 and 12 months. Vitamin D levels is going to be performed at 0, 4, 12 month interval. MRI is going to be done at the beginning and end of trial.The number of relapses and the physical disability will be calculated through the Expanded disability status scale (EDSS).
Publication(s):
Mahmoud Abedini, Mazandaran University of Medical Sciences, Islamic Republic of Iran
Vitamine D in Multiple Sclerosis
Dose: 50 000 IU/w vitamin D3 versus placebo, for 12 months
Estimated Enrollment: 240
Start Date: March 2013
Completion Date: October 2014
NCT Number: NCT01768039
Brief summary:
Two hundred and forty patients with multiple sclerosis who met the study criteria will be enrolled in this randomized double blind placebo-controlled clinical trial.
They will randomly assigned to placebo or vitamin D treatment group. The total time of study is 52 weeks and the vitamin D group will be treated by weekly 50000 International unit(IU) vitamin D, while the other group will receive weekly placebo. The annual relapse rate and EDSS will be compared at baseline, month 6 and 12.
Publication(s):
Ellen M Mowry, MD, MCR Johns Hopkins University
Pharmacokinetics of Vitamin D in Multiple Sclerosis and in Health
Dose: 5000 IU/day of vitamin D3, for 90 days
Estimated Enrollment: 57
Start Date: November 2010
Primary Completion Date: March 2014
NCT Number: NCT01667796
Brief summary:
This is a pilot study of oral vitamin D supplementation to determine if patients with Multiple Sclerosis (MS) and healthy individuals attain a similar increase in serum 25-hydroxyvitamin D levels. The investigators will also assess whether the immunologic or relevant gene expression response to oral vitamin D supplementation differs in patients with MS and healthy controls.
Vitamin D Supplementation in Multiple Sclerosis
Dose: 600 IU/day versus 5 000 IU/day of vitamin D3, for 2 years
Estimated Enrollment: 172
Start Date: March 2012
Completion Date: March 2015
NCT Number: NCT01490502
Brief summary:
In this clinical trial, patients with relapsing-remitting MS will receive high-dose or low-dose oral vitamin D in addition to an approved therapy for MS, glatiramer acetate (Copaxone®, Teva Pharmaceutical Industries). Patients will be evaluated for two years, and the effect of high-dose vitamin D supplementation on the rate of MS attacks and on the number of new lesions and change in brain volume on MRI will be determined. Establishing this association will have major implications for the treatment of individuals with MS throughout the world.
Publication(s):
Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, Gourraud PA, Brenneman D, Owen MC, Qualley P, Bucci M, Hauser SL, Pelletier D. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012 Aug;72(2):234-40. doi: 10.1002/ana.23591.
Raymond Hupperts, MD, Dept of Neurology, Orbis Medical Center Sittard, Maastricht University, The Netherlands
Supplementation of VigantOL® Oil Versus Placebo as Add-on in Patients With Relapsing Remitting Multiple Sclerosis Receiving Rebif® Treatment (SOLAR)
Dose: 14 000 IU/day of vitamin D versus placebo, for 2 years
Estimated Enrollment: 358
Start Date: February 2011
Primary Completion Date: March 2014
NCT Number: NCT01285401
Brief summary:
The drug being tested is called Vigantol® oil - a very effective form of Vitamin D hormone supplement (cholecalciferol). Low levels of Vitamin D have been described to be associated with a higher risk of developing Multiple Sclerosis (MS), and it is known that up to 90% of patients with Multiple Sclerosis have Vitamin D deficiency.
Rebif® is known to be an effective treatment for slowing down the progression of MS. The purpose of this research trial is to evaluate if Vigantol® oil on top of Rebif® has any benefit on the progression of MS compared to Rebif® and placebo.
Disease activity will be assessed by clinical examination and Magnetic Resonance Imaging (MRI). The planned treatment duration for each study participant is 96 weeks, and the study consists of a total of 12 visits.
During the study, the participant will undergo physical examination, neurological assessments, safety assessments, blood tests and urinalysis (including pregnancy tests).
Publication(s):
Vitamin D and the Stress-axis in MS
Dose: 4 000 IU/d vitamin D3, for 4 months
Estimated Enrollment: 80
Start Date: August 2014
Completion Date: March 2015
NCT Number: NCT02096133
Brief summary:
Patients with multiple sclerosis (MS) have an increased risk of developing a major depression. The investigators observed a protective effect of high vitamin D levels on the risk of depression in MS. This might be driven by the effect of vitamin D on the stress-axis. Therefore, the main goal of the present study is to assess whether high dose vitamin D supplementation results in a suppression of the stress-axis, as measured by decreased levels of cortisol.
Publication(s):
Anne-Sophie Jean Deleglise, Merck Serono S.A.S, France
A Multicentre Study of the Efficacy and Safety of Supplementary Treatment With Cholecalciferol in Patients With Relapsing Multiple Sclerosis Treated With Subcutaneous Interferon Beta-1a 44 µg 3 Times Weekly (CHOLINE)
Dose: 100 000 IU/twice monthly of vitamin D3 versus placebo, for 2 years
Estimated Enrollment: 250
Start Date: January 2010
Primary Completion Date: July 2014
NCT Number: NCT01198132
Brief summary:
The aim of this multicentre, randomised, double-blind, placebo-controlled study is to evaluate the efficacy and safety of supplementary treatment with cholecalciferol (vitamin D3) in subjects with relapsing multiple sclerosis (RRMS) treated with subcutaneous (s.c.) interferon beta-1a 44 µg (Rebif) 3 times weekly. The subjects will be divided into 2 groups, one receiving cholecalciferol 100,000 IU twice monthly along with Rebif treatment and the other group will be on placebo along with Rebif treatment. A total of 200 subjects will be recruited in 20-30 centres in France.
Publication(s):
Jan-Markus Dörr, Charite University, Berlin, Germany
Efficacy of Vitamin D Supplementation in Multiple Sclerosis (EVIDIMS)
Dose: 20 000 IU/2d 400/2d of vitamin D3 versus 400/2d, for 18 months
Estimated Enrollment: 80
Start Date: December 2011
Completion Date: December 2016
NCT Number: NCT01440062
Brief summary:
Examination of efficacy, safety and tolerability of vitamin D3 in the treatment of Multiple Sclerosis (MS).
Publication(s):
Dörr J, Ohlraun S, Skarabis H, Paul F. Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS Trial): study protocol for a randomized controlled trial. Trials. 2012 Feb 8;13:15. doi: 10.1186/1745-6215-13-15.
Helmut Butzkueven, MD, PhD, Department of Neurology, The Royal Melbourne Hospital, Australia
Preventing the risk of Multiple Sclerosis using Vitamin D in patients with a first demyelinating event in Australia and New Zealand (PrevANZ)
Dose: Vitamin D3 1 000 IU/d, 5 000 IU/d, 10 000 IU/d or placebo, for 48 weeks
Estimated Enrollment: 240
Start Date: December 2012
Completion Date: 2017
ACTRN 12612001160820
Brief summary:
Phase IIb Randomized, Double-Blind, Placebo-Controlled, Dose Ranging Trial to determine the safety and efficacy of Vitamin D3 in preventing the risk of MS in Patients with a first demyelinating event.
Publication(s):
Merja Soilu-Hanninen, MD, PhD, neurologist, University of Turku, Department of Neurology, Turku, Finland - published
Colecalciferol as an Add-on Treatment to Interferon-beta-1b for Treatment of Multiple Sclerosis (MS)
Dose: 20 000 IU/week of vitamin D3 versus placebo for 1 year
Estimated Enrollment: 70
Start Date: March 2008
Completion Date: March 2014
NCT Number: NCT01339676
Brief summary:
This is a multi-centre, double blind, randomised, placebo controlled, parallel group, phase 4 pilot study investigating colecalciferol (vitamin D3) as an add-on treatment to subcutaneously administered interferon-beta-1b in relapsing-remitting multiple sclerosis patients.
Publication(s):
Soilu-Hänninen M, Aivo J, Lindström BM, Elovaara I, Sumelahti ML, Färkkilä M, Tienari P, Atula S, Sarasoja T, Herrala L, Keskinarkaus I, Kruger J, Kallio T, Rocca MA, Filippi M. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012 May;83(5):565-71. Epub 2012 Feb 22.
Aivo J, Lindsröm BM, Soilu-Hänninen M. A Randomised, Double-Blind, Placebo-Controlled Trial with Vitamin D3 in MS: Subgroup Analysis of Patients with Baseline Disease Activity Despite Interferon Treatment. Mult Scler Int. 2012;2012:802796. doi: 10.1155/2012/802796. Epub 2012 Aug 5.
Merja Soilu-Hanninen, MD, PhD, neurologist, University of Turku, Department of Neurology, Turku, Finland
Colecalciferol as an Add-on Treatment to Subcutaneously-Administered Interferon-beta-1b for Treatment of Multiple Sclerosis (MS)
Dose: 20 000 IU/week of vitamin D3 versus placebo for 1 year
Enrollment: 70
Start Date: March 2008
Completion Date: March 2011
NCT Number: NCT01432704
Brief summary:
This is a one-year multi-centre, double blind, placebo controlled, randomized trial investigating oral vitamin D3 (Colecalciferol) as an add-on treatment to interferon-beta-1b for Multiple Sclerosis (MS). Not less than one month after initiation of therapy with interferon beta 1b, MS patients will be randomised to once weekly treatment with peroral colecalciferol capsules (Dekristol®, Swiss-Caps, Switzerland) containing 0.5 mg of vitamin D3, or to once weekly peroral treatment with matching placebo. The hypothesis is that vitamin D suppresses clinical and MRI activity of MS.
Publication(s):
Margitta T Kampman, MD, PhD, University Hospital of North Norway - published
Can Vitamin D Supplementation Prevent Bone Loss in Persons With Multiple Sclerosis
Dose: 20 000 IU/week of vitamin D3 versus placebo for 2 year
Estimated Enrollment: 80
Start Date: January 2008
Completed
NCT Number: NCT00785473
Brief summary:
Supplementation with 20,000 IU vitamin D(3) a week did not prevent bone loss in this small population. Larger studies are warranted to assess the effect of vitamin D on bone health in persons with MS.
Publication(s):
Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler. 2012 Aug;18(8):1144-51. doi: 10.1177/1352458511434607.
Steffensen LH, Jørgensen L, Straume B, Mellgren SI, Kampman MT. Can vitamin D(3) supplementation prevent bone loss in persons with MS? A placebo-controlled trial. J Neurol. 2011 Mar 13.
Joost Smolders, MD, Maastricht University Medical Center - published
Vitamin D3 Supplementation and the T Cell Compartment in Multiple Sclerosis (MS)
Dose: 20 000 IU/day of vitamin D3 for 3 months in winter
Enrollment: 15
Completion Date: July 2010
NCT Number: NCT00940719
Brief summary:
Twelve week supplementation of high dose vitamin D3 in RRMS patients was well tolerated and did not induce decompensation of calcium metabolism. The skewing towards an anti-inflammatory cytokine profile supports the evidence on vitamin D as an immune-modulator, and may be used as outcome measure for upcoming randomized placebo-controlled trials.
Publication(s):
Smolders J, Peelen E, Thewissen M, Cohen Tervaert JW, Menheere P, Hupperts R, Damoiseaux J. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One. 2010 Dec 13;5(12):e15235.
Jodie M Burton, MD, St. Michael's Hospital, University of Toronto - published
Safety Trial of High Dose Oral Vitamin D3 With Calcium in Multiple Sclerosis (VitD4MS)
Dose: on average14 000 IU/day of vitamin D3 for 1 year
Enrollment: 49
Start and Completion Date: July 2006 - February 2008
NCT Number: NCT00644904
Brief summary:
High-dose vitamin D (approximately 10,000 IU/day) in multiple sclerosis is safe, with evidence of immunomodulatory effects. CLASSIFICATION OF EVIDENCE: This trial provides Class II evidence that high-dose vitamin D use for 52 weeks in patients with multiple sclerosis does not significantly increase serum calcium levels when compared to patients not on high-dose supplementation. The trial, however, lacked statistical precision and the design requirements to adequately assess changes in clinical disease measures (relapses and Expanded Disability Status Scale scores), providing only Class level IV evidence for these outcomes.
Publication(s):
Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R, Gagne D, D'Souza C, Ursell M, O'Connor P. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010 Jun 8;74(23):1852-9.
Kimball 2008 - published
Self-prescribed high-dose vitamin D3: effects on biochemical parameters in two men.
Dose:
Enrollment: 2
Results:
The first evidence of a potential adverse effect was that urinary calcium:creatinine ratios showed an increasing trend, which preceded serum calcium concentrations above the reference range (2.2-2.6 mmol/L). His serum 25(OH)D concentration was 1126 nmol/L when total serum calcium reached 2.63 mmol/L. He stopped vitamin D3 supplementation at this point. Two months later, all biochemistry values were within reference ranges; serum 25(OH)D concentrations fell by about one-half, to 656 nmol/L. These results help to clarify the human response to higher intakes of vitamin D3. Close monitoring of biochemical responses confirmed that an increase in urinary calcium:creatinine ratio precedes hypercalcaemia as serum 25(OH)D concentrations rise.
Publication(s):
Kimball S, Vieth R. Self-prescribed high-dose vitamin D3: effects on biochemical parameters in two men. Ann Clin Biochem. 2008 Jan;45(Pt 1):106-10.
Kimball 2007 - published
Safety of vitamin D3 in adults with multiple sclerosis.
Dose: 2.800-28.000 IU/week of vitamin D3, for 28 weeks
Enrollment: 12
Results:
Patients' serum 25(OH)D concentrations reached twice the top of the physiologic range without eliciting hypercalcemia or hypercalciuria. The data support the feasibility of pharmacologic doses of vitamin D3 for clinical research, and they provide objective evidence that vitamin D intake beyond the current upper limit is safe by a large margin.
Publication(s):
Kimball SM, Ursell MR, O'Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007 Sep;86(3):645-51.
Mahon 2003 - published
Cytokine profile in patients with multiple sclerosis following vitamin D supplementation.
Dose: 1 000 IU/day of vitamin D3, for 6 months
Enrollment:
Results:
Vitamin D supplementation of MS patients for 6 months was associated with increased vitamin D status and serum TGF-beta 1.
Publication(s):
Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003 Jan;134(1-2):128-32.
Goldberg 1986 - published
Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D.
Dose: 5000 IU/day of vitamin D3, for one to two years
Enrollment:
Results:
The dietary regimen may offer a new means of controlling the exacerbation rate in MS, at least for younger patients. The results tend to support a theory of MS which states that calcium and magnesium are important in the development, structure and stability of myelin.
Publication(s):
Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses. 1986 Oct;21(2):193-200.
FaceBook Vitamin D and MS
Page last edited: June 12, 2016
Terms of Use
